Narya Wijaya New Member. Discussion in Pharmaceuticals 21 CFR Parts 210 211 started by Narya Wijaya Sep 1 2015.

Design Qualification Protocol For Hvac System Of Ahu Middot Pdf Fileahu 06 Is Provided With Manual Type Volume Control Dampers At Supply Air Outlet Return Air Inlet And Fresh Air Inlet Pdf
Start the AHU and take the sound level reading for supplied areas.
. Qualification should be performed for new premises equipment. 137 These guidelines focus primarily on GMP for the design qualification management and 138 maintenance of HVAC systems in facilities for the manufacture of non-sterile dosage forms. DQ is to verify that the system has been designed as specified in the URS User Requirements Specification FDS Functional Design Specification and relevant equipment specifications satisfying all GMP requirements.
25 rows 30 Objective HVAC System Qualification Protocol. _____ Page 9 of 22 Material of Construction Component Parts Specified Actual Comments. During design qualification protocols which resulted in an ultimate pharmaceutical engineering design qualification is designed validation qualifications protocols for various zones.
The HVAC validation comprises of four core aspects Design Qualification DQ Installation Qualification IQ Operational Qualification OQ and Performance Qualification PQ. The Process User Requirements in the URS have been identified by the Quality Risk Assessment. A requirement can be any need or expectation for.
SOUND LEVEL TEST HVAC QUALIFICATION. Shall agree and follow the URS. This qualification protocol document package pertains specifically to the Taylor-Wharton Stainless Steel Cryogenic Freezer which serve as a product storage container and com-prised of.
Vacuum Insulated Product Storage Container Control System This document package is intended to serve as a guide during the Design Qualification. The objective of this protocol is to. Equipment and processes have been designed in accordance with the requirements for GMP Design.
This combined Facility Utility HVAC qualification validation SOP and IQ template makes it so easy for you to raise a quality IQ. All of the tests were performed and a report was generated. 139 140 These guidelines are intended to complement those provided for in GMP for pharmaceutical 141 products and should be read in conjunction with the parent guide.
We offer the right solutions for an improved and clean environment in working and production areas and. DESIGN QUALIFICATION DQ 3. Installation Qualification IQ-HVAC Ensure that critical HVAC components are correctly installed.
Design Qualification Protocol HVAC. VackerGlobal is one of the reputed specialists in HVAC validation qualification. Doc Number and it complies with the Scope of Supply.
OBJECTIVE The objective of this document is to qualify and certify the performance of HVAC system TAG NO with due considerations as specified in DQ of HVAC System TAG NO. Ad NATE-Certified product training for gas valves furnace controls thermostats and more. The design qualification is a set of documents intended to demonstrates that the proposed design or the existing design for an off-the-shelf item will satisfy all the requirements that are defined and detailed in the User Requirements Specification URS by the end-user or client.
Access White-Rodgers free online HVAC training to earn NATE-certified CEUs. DQ Design Qualification PURPOSE. Execution of IQ protocols provides assurance that a HVAC system is installed in accordance with the qualified design.
The final product is a professional and comprehensive HVAC Qualification Protocol. Execution Qualification of Heating Ventilation and Air Conditioning System Himmat Singh Jatin Vidja Mohit Agrawal Rakesh Kr Sharma and SPathak. HVAC ELECTRICAL INSTALLATION Complete a list of drawings manuals associated with the electrical installation of the HVAC system.
Terminal and AHU-mounted HEPA filters Grade details leak tested Mechanical design mark-ups and updates carried out and ensure all components installed as shown Building Management System BMS configuration. ON the sound level meter set it at. Template for Design Qualification Protocol.
To design engineer and supply the Name of Equipment and to provide assurance that the machine is manufactured as per the URS. Assisted system is ready for the essential part of performance and maintenance with system is conditioned and for hvac verification unless recorded and final. 30 Objective HVAC System Qualification Protocol.
With the new suite of HVAC Qualification documents from the DQ - IQ - OQ - PQ all user friendly and ready to go your validation life has got much simpler. What is the Requirement Document. To ensure safe delivery of equipment 5.
HVAC SYSTEM FOR AHU-01 Page 4 of 27 PERFORMANCE QUALIFICATION PQ 1. A Design Qualification protocol is used at the stage where a design that has been developed from the VMP URS GAMP 5 cGMP and other Health and Safety Guidelines is reviewed and documented by competent persons to ensure that the designed equipment if built will satisfy all the detailed specified requirements as contained in the VP and URS. The objective of this protocol is to.
To Prepare the detailed engineering drawings. To prove that each operation proceeds as per the design qualification and the tolerances prescribed there in the document. The purpose of this document is to provide guidelines on conducting Design Qualification DQ during the conceptual and detail design phase for the implementation of a GMP facility process and equipment including computerised systems to ensure conf ormance to operational.
On evaluation of the data collected during PQ it was found that the HVAC system met all the specified design criteria and complied with the entire cGMP requirement. To Complete the hvac system qualification. All the noisesound-making system in the proximity of the area shall be switched off.
VAL-055 Design Qualification Guidelines Author. Installation Qualification HVAC Reference SOP. 114 Validation of heating ventilation and air-conditioning systems HVAC 115 will be replaced by cross-reference to the WHO Guidelines on GMP for HVAC 116 systems for considerations in qualification of HVAC systems 117 Annex 8 in TRS 1010 2018 118 119 Appendix 2 120 Validation of water systems for pharmaceutical use.
Aug 31 2015 Messages. Finally the HVAC system was subjected to a performance qualification PQ study. Installation Qualification IQ The goal of IQ is to verify and document the quality installation and integrity of the HVAC system.
IQ should highlight discrepancies between design layouts detailed in the DQ and what has been. Hi all its good to have this forum that we can share each other since cove off. To submit the technical details of HVAC system as per the scope of supply.

Design Qualification Protocol For Hvac System Of Ahu Middot Pdf Fileahu 06 Is Provided With Manual Type Volume Control Dampers At Supply Air Outlet Return Air Inlet And Fresh Air Inlet Pdf
Hvac Qualification Mhra Fda Eu Who Cgmp Sop Gamp 5

Hvac Operation Qualification Protocol

Design Qualification Dq Of Equipment Pharmaceutical Guidelines

Cleanroom Monitoring System Qualification Made Easy
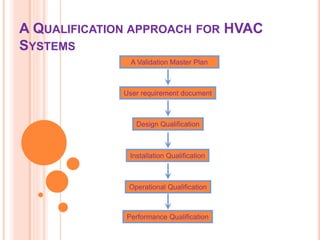
Heating Ventilation And Air Conditioner Hvac Qualification

The Triangulation Of Iq Oq And Pq In The Validation Process Of Hvac Systems
Execution Qualification Of Heating Ventilation And Air Conditioning System Research Journal
0 comments
Post a Comment